| RUS | UKR | ENG |
 |
Biletskij Evgenij Jurijovich |
DonNTU |
| Faculty of ecology and chemical technology | Dissertation | |
| Speciality: Chemical technology of high molecular substances | Library | |
| Theme of master's work: Development of the well-stirred tank reactor automated control system | Links | |
| E-mail: houseofpain@inbox.ru | Search results |
Summary
Urgency of theme
Trotyl is a basic component of many industrial explosives. Main advantage of trotyl is that being strong blasting explosive, it has low sensitivity to mechanical actions. It simplifies production of explosives on its base.
Trotyl produced by nitrating of toluene by a mixed sulfuric and nitric acid (nitrosulphuric acid). The majority of apparatus that used now in the production of trotyl consists of several nitrators and separators. The process is multiphase with the countercurrent motion of components between the reactors. Nitrator is the volumetric type reactor with an impeller (mechanical or pneumatic).
Existing schemes for toluene nitration practically are not computerized – each nitrator in a stage is equipped with a temperature sensing device, that at an increase of temperature above the certain level dumps reaction mixture in an emergency container with cold water.
Purpose and problems of present work
The purpose of the present work is the development of mathematical model of toluene nitration in a stage of well-stirred tank reactors with various schemes of a reagents motion between stages. The continuous and periodic reactors with different temperature modes (an adiabatic reactor, an isothermal reactor, a reactor with the attemperation) will be viewed. On the basis of the gained mathematical model the search of optimum requirements for process conditions – a temperature mode, a rate of flux and concentrations of reagents will be carried out. To optimize the model it is planned to apply a genetic algorithm as the most perspective method for multiparameter multiextreme function optimization. Effects of calculation of optimum parameters values can be applied to develop an automated management system for the toluene nitration process.
The brief description of toluene nitration model

The schematic representation of the first stage of toluene nitration – nitronium catione associates with an aromatic ring forming π-complex; then NO2 group is displaced in an ortho-standing against methyl group with the formation of α-complex; then Í+ ion is eliminating.
The purpose of the model is to provide the calculation of the composition and temperatures of a reaction mixture at the reactor outfeet (for a reactor with the continuous duty) or the concentration distribution of reagents in an intermixture and temperatures of an intermixture in time (for a batch reactor).
Mass balance
Generally the change of reagents concentration in a reaction mixture is expressed by the equation:
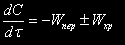
where Wïåð is the change of reagent concentration owing to the hydraulic transfer;
Wõð is the change of reagent concentration owing to the chemical reaction;
For a periodic reactor Wïåð equal to null, for a continuous reactor 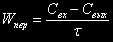 . The change of reagent concentration owing to a chemical reaction for a second-order irreversible reaction
. The change of reagent concentration owing to a chemical reaction for a second-order irreversible reaction
À + Â –> Ñ + D
is defined by the equation:
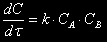
where k is a rate constant of a chemical reaction
ÑÀ, ÑÂ are molar concentrations of reagents A and B respectively.
Without oxidative processes and formation of the asymmetric nitrotoluenes the scheme of nitrating looks as follows:
C7H8 + HNO3 –> C7H7 NO2 + H2O
C7H7NO2 + HNO3 –> C7H6 (NO2)2 + H2O
C7H6(NO2)2 + HNO3 –> C7H5(NO2)3 + H2O
Then changes of concentration for each reagent will be equal (for a periodic reactor):
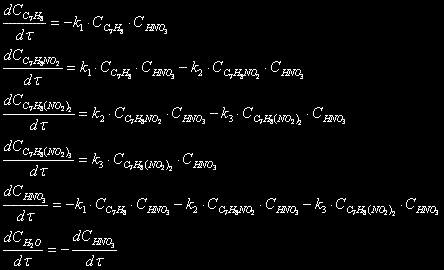 (1)
(1)
By the solution of all these equations it is possible to define a concentration distribution of reagents in time.
The nitration reaction proceeds in heterogeneous conditions i.e. there are two phases in the reactor: organic, initially containing toluene, and mineral containing a nitrosulphuric acid and dissolved toluene. The diffusion rate of components through the interfacial area can be described by the equation
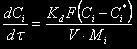
where Kd is a mass-transfer rate coefficient;
F is a interfacial area of phases;
Ñi* is equilibrium concentration of the i-th component;
Ìi is molar mass of the i-th component;
The equilibrium concentration of a reagent can be expressed as
Ñi* = mi Ci
where mi is a distribution coefficient for the i-th component between mineral and organic phases.
The nitrating agent in an intermixture of concentrated sulfuric and nitrogenous acids is the nitronium ion NO2+, therefore in the equations (1) instead of nitric acid concentration it is necessary to substitute NO2+ concentration. Direct determination of NO2+ concentration in an intermixture is impossible. It is possible to count it in two ways:
1)On the basis of empirical data about a degree of dissociation of nitric acid (the degree of dissociation depends on the relation of concentrations of sulphuric acid, nitric acid and water):
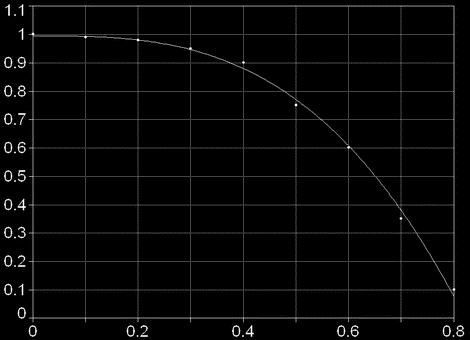
Dependence of the degree of HNO3 conversion in NO2+ from relation H2SO4/H2O
2)On the known equilibrium constant for HNO3 dissociation :
Heat balance
The change of temperature in a reactor occurs owing to temperature differences of entering and getting out streams, thermal effects of chemical reactions and a heat transfer between a reaction mixture and environment and is described by the equation:
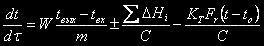
where W is a rate of flux of an intermixture;
tâûõ is the temperature of an intermixture on the reactor outfeet;
tâõ is the temperature of an intermixture on an inlet in a reactor;
ΔÍi is the heat effect of the i-th reaction.
ÊÒ is the heat transfer rate coefficient;
Fr is the surface of the reactor.
t is the temperature of an intermixture in the reactor;
tî is the ambient temperature.
C is the heat capacity of an intermixture in a reactor.
The first term of this equation shows the change of temperature owing to transfer of heat by entering and getting out streams, second one shows the change of temperature owing to heat effects of reactions and the third is the heat transfer to environment.
For the batch reactor the first term of this equation will be equal to null (there are no entering and getting out streams). For the adiabatic reactor (without heat exchange with the environment) the last term of this equation will be equal to null.
Present results
On the present time the model of a batch adiabatic well-stirred tank reactor has been realized. The concentration distribution of components and temperatures has been gained. It is planed to expand the model with the help of equations featuring toluene oxidizing processes by the nitric acid, nitration with the formation of by-products i.e. the asimmetric nitrotoluenes; the equations for the description of heat exchange with the environment. It is also planned to realize model of a continuous reactor and the model of a stage of reactors with a uniflow and countercurrent motion of reagents between stages.
- Basil T.Fedoroff «Encyclopedia of explosives and related items» Pacarinny Arsenal, Dover, New Jersey, USA, 1960.
- Tadeuzs Urbanski «Chemistry and technology of explosives» Pergamon Press, PWN - Polish Scientific Publishers, Warszava, 1962.
- Ben J.McCoy Giridhar Madras «Chemical Kinetics in Dispersed-Phase Reactors» INTERNATIONAL JOURNAL OF CHEMICAL REACTOR ENGINEERING, Volume1, 2003.