Abstract |
Abstract:
"The kinetics of the oxygen exchange in cuprate barium-yttrium, alloyed by the samarium"
Kirichenko N.
The superconductivity is an effect of electric resistance disappearing in some metals, alloyes and chemical compounds under low temperatures.
Cuprate barium-yttrium is one of the high-temperature superconductors. Because of its properties these materials are perspective in different fields of technology. They would be able to be used in powerengineering, chemistry, electric equipment, etc.
The properties of this compound oxide depend on oxygen content in it, the character of the oxygen entry and strenght of oxygen connection in cuprates. So, the aim of this work was to research oxygen behaviour in YBa2Cu3Ox, if the oxygen had been substituted by the samarium.
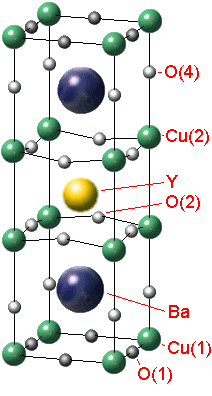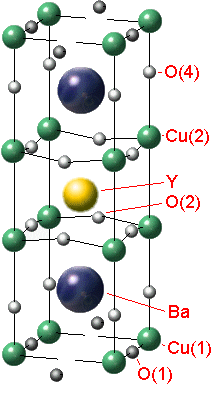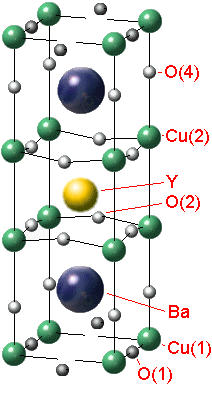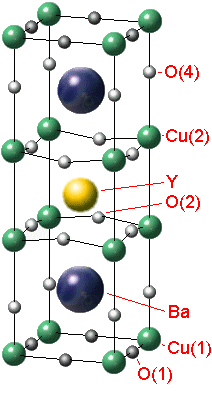
Let's invastigate the properties of the YBa2Cu3Ox. There is a structure of the crystal lattice of this material (fig. 1).

Fig. 1 - The YBa2Cu3Ox tetragonal-to-orthorhombic structure transition
It's common to think that the crystal YBCO has purely ionic coupling as the crystal NaCl. The special role in the structure of the crystal lattice YBCO has '''O. The ion '''O isn`t available at the stoichiometrical content of YBa2Cu3O6 (x=6) and if x is 7 (for YBa2Cu3O7) all vacancies of '''O are completely full. The oxygen stoichometry is easy to regulate by heating the crystal at the definite pressure of oxygen with the following quenching. The oxygen content in the crystal influences on its electrophysical properties.The crystal YBCO has a metal conductivity under enough high temperature. If the temperature is decreasing there is a transition into dielectrics or superconductors depending on the value x.
The oxygen stoichiometry influences on the superconducting properties of YBa2Cu3O7-x mainly. It is typical for them to exist of two polymorphic structures: there are tetragonal (x > 0,5) and rhombic (orthorhombic) (0 < x < 0,5) structure.
The synthesis of ceramic high-temperature superconducting materials is carried out at the temperature over 9000C. The oxygen content at these temperatures is 6,2 atoms per formal one and the quenching of the sample causes the formation of YBa2Cu3O7-x tetragonal nonconducting structure.
When YBa2Cu3O7-x is rich of oxygen it is taking place the transition from tetragonal into orthorhombic structure (x < 0,5). The temperature of this transition depends on oxygen partical pressure.
The phase transition is taken place if the temperature and the content (the oxygen content) of phase have changed. The changing of the structure is insignificant.
When the transition from tetragonal to orthorhombic structure is taking place in addition to the changing of the oxygen stoichiometry the rebuilding of the oxygen underlattice can be found.
As for all mentioned above, the properties of HTSC-materials depend on their oxygen content. So, the scientists need to deal with the purpose to find HTSC of the new generation. The main direction of this research is to build new compound - chains systems (CuO2)n with different layer configurations. It could be reached by the selection of new stoichiometric contents, t.e. by the partical substitute of barium to its izoelectronic analogue - Sm.
We have done the following experiments. The cuprate samples YBa2-zSmzCu3Ox were made from the charge of yttrium oxide Y2O3, cuprium oxide CuO, samarium oxide Sm2O3 and barrium carbon BaCO3. The compounds were splintered with great care in an agatium plate under an etilliquid layer. The samples were produced according to ceramic technology. The cuprates were being synthesed during 20 hours at 9300C with two intermediate crushings. Then the samples were sintered in the air at 9400C.
After this we did the thermocyclization. It is the heating and cooling of the samples in turns from 20 to 9000C in the gravimetric plant (fig. 2).
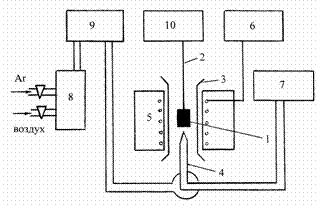
Fig. 2 - The gravimetric plant
The samples (3-4 g) were hung up on the platinum thread inside vertical silica reactor with two sections. As the result of this research of YBa1,9Sm0,10Cu3Ox oxygen content there has been discovered the anomal oxygen relaxation provided by the cuprate thermocyclization. There was some gysterezis at the area of the tetragonal-to-orthorhombic phase transition (fig. 3,4).
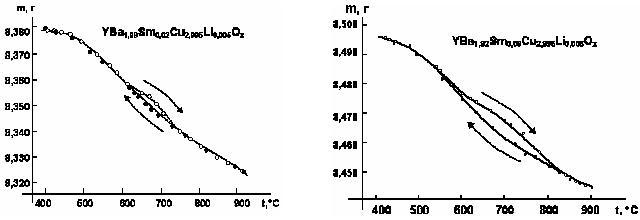
Fig. 3, 4 - The gysterezis of the YBa2-hSmhCu3Ox samples
But there wasn't any gysterezis for the samples, which hadn`t been alloyed by samarium.
Because of this we decided to build the cooling and heating curves (fig. 5).
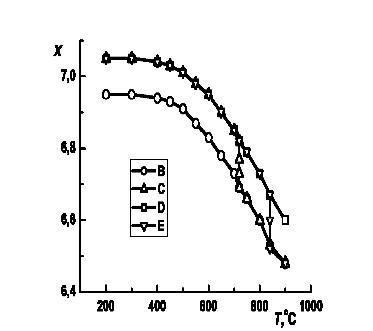
Fig. 5 - The YBa1,90Sm0,10Cu2,995Li0,005Ox changing of oxygen content
When the samples were cold from 9000C the oxygen content x was changing along the curve B. But the situation changed when the samples had been kept at certain temperatures. We observed the "deviation" of these curves, which we could explain as slow oxidation in the case of the cooling and as reduction in the case of samples cooling.In conclusion we may say, the analysis of the experimental data has confirmed once again the presence of 2 forms of nonstoichiometrical oxygen, which exhibit themselves in the temperature's range (740-8400C). We have also done the radiographical analysis of the samples. As a result of it we have got the diffractograms (fig. 6), using which we were able to calculate the crystal lattice parameters (table).
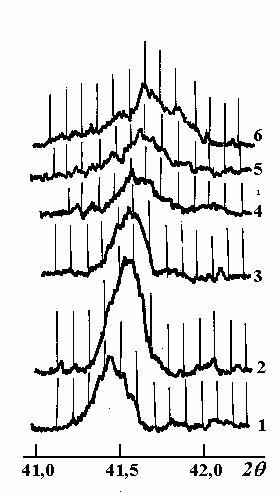
Fig.6 - The different oxygen content samples` diffractograms
Table
The YBa2-hSmhCu2,995Li0,005Ox crystal lattice parameters
Containt |
The crystal lattice parameters | ||
a | b | c |
|
| YBa2Cu2,995Li0,005Ox | 4,296 |
4,207 |
12.888 |
| YBa1,98Sm0,02Cu2,995Li0,005Ox | 4,296 |
4,217 |
12,888 |
| YBa1,96Sm0,04Cu2,995Li0,005Ox | 4,295 |
4,228 |
12,885 |
| YBa1,94Sm0,06Cu2,995Li0,005Ox | 4,295 |
4,235 |
12,885 |
| YBa1,92Sm0,08Cu2,995Li0,005Ox | 4,294 |
4,245 |
12,881 |
| YBa1,90Sm0,10Cu2,995Li0,005Ox | 4,296 |
4,267 |
12,888 |
Abstract |